22+ nernst equation calculator
Some of my friends had challenges like I did with the Nernst equation so here is a short video of a Arduino calculator I built that may help you understan. The following example shows how the Nernst equation may be used to calculate the potential of an electrochemical cell at non-standard conditions.
Electrochemistry
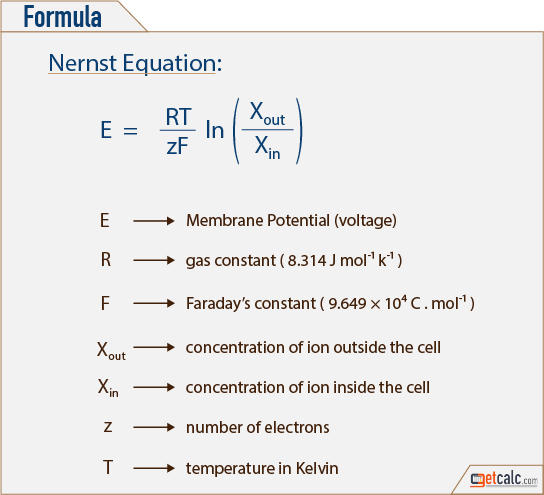
The Nernst equation for 298 Kelvin can be represented as follows.

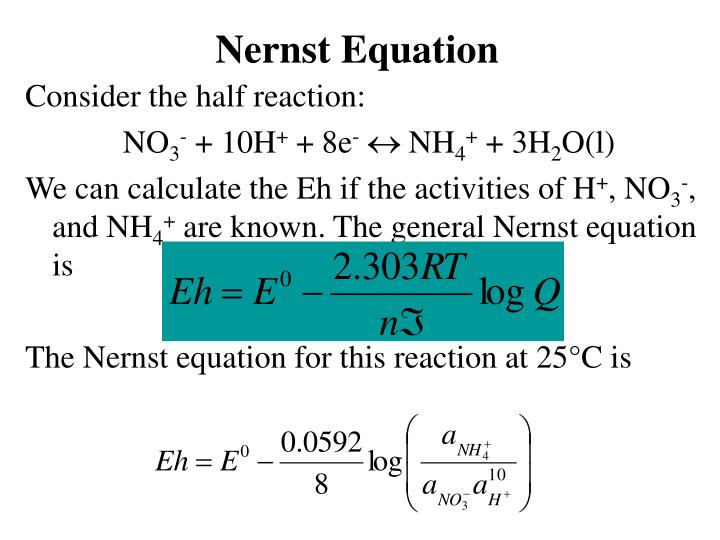
. So we have zero is equal to the. E E₀ - RTzF ln red ox where. 22 nernst equation calculator Senin 31 Oktober 2022 The Nernst equation is E is equal to E zero minus 0592 over n times the log of Q.
Easy is good so we basically want to force the quadratic equation. Well at equilibrium at equilibrium E is equal to zero so we plug that in. In the input field enter the standard half-cell reduction potential chemical activity for the reductant and oxidant.
E -00496 T log 10 pO. The Nernst equation works for half-cell and full cell reactions. This calculator will allow the calculation of the expected.
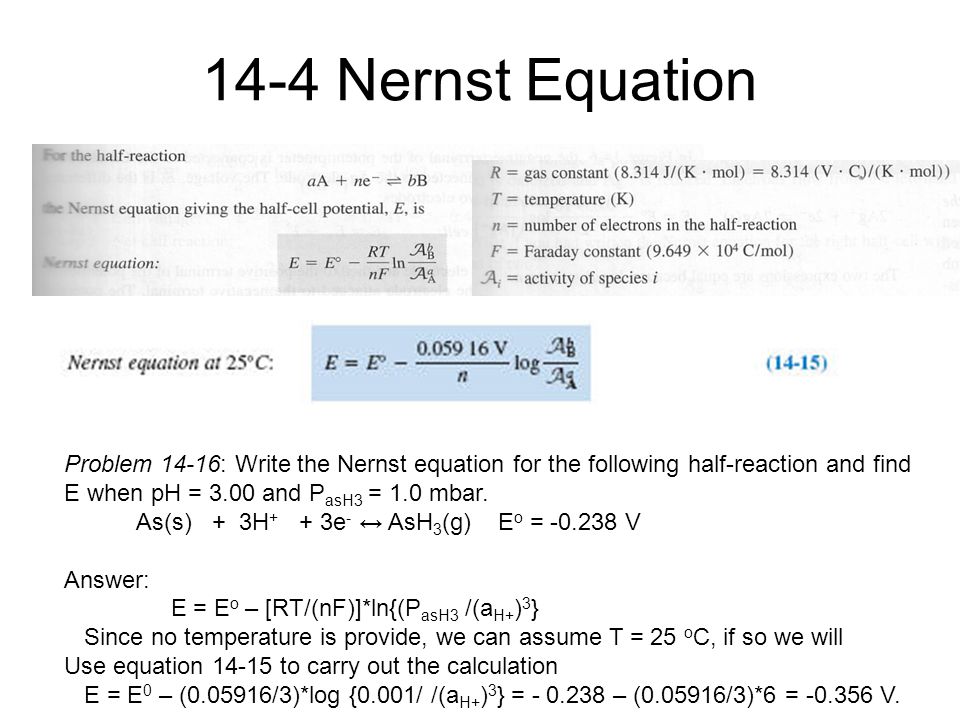
The Nernst equation is E is equal to E zero minus 0592 over n times the log of Q. In electrochemistry the Nernst equation is a chemical thermodynamical relationship that permits the calculation of the reduction potential of a reaction half-cell or full cell reaction from the. E 0021544 T ln p O 2 p O 2 And using log10.
An equation that relates the Gibbs free. E₀ -- Standard Reduction. If oxygen is to be measured using a zirconia ceramic electrolyte and atmospheric air with pO 2 0209 as a reference this equation simplifies to.
The following is the procedure how to use the Nernst equation calculator Step 1. The Nernst equation which measures electrical energy is used to find the cell potential or voltage of a system. The formula was developed by Nobel Prize winner Walther Nernst and is.
The following formula is used to calculate the reduction potential of a cell reaction. PO 2 0209 exp-46421 ET The equation. E E0 00592n log_10 Q Hence as per the Nernst equation the potential of the electrochemical cell depends on the.
The equation may be re-arranged to allow calculation of the emf from a known reference concentration and a measured oxygen concentration. E 00496 T log 10 p O 2 p O 2 The output of both equations is in mV. E -- The reduction potential expressed in volts.
E E0 RTZf ln Q. Electric Potential Calculator. An equation in which one side is a perfect square trinomial can be easily solved by taking the square root of each side.
The Nernst equation is often used to calculate the cell potential of an electrochemical cell at any given temperature pressure and reactant concentration. The equation was introduced by a.

Write The Nernst Equation And Emf Of The Following Cells At 298 K I Mg S Mg 2 0 001 M Cu 2 0 0001 M Cu S Ii Fe S Fe 2 0 001 M H 1m H2 G 1bar Pt S Iii
At The Calculated Reversal Using The Nernst Equation Potential For An Ion Does The Current Measured Depend On The Ion Or Temperature Or The Organism The Channel Is From Or Cannot Be
How To Calculate And Solve For Nernst Equation Corrosion Nickzom Blog

Statistics In Analytical Chemistry Excel

Write The Nernst Equation And Emf Of The Following Cells At 298 K I Mg S Mg 2 0 001 M Cu 2 0 0001 M Cu S Ii Fe S Fe 2 0 001 M H 1m H2 G 1bar Pt S Iii

Nernst Equation Example Concentrations Youtube
Write Nernst Equation And Calculate The Emf Of The Following Cell At 298 K Sarthaks Econnect Largest Online Education Community
Nernst Equation Calculator
Chemistry Analytical Chemistry Fall Ppt Video Online Download

Nernst Equation Calculator

10 Best Free Online Nernst Equation Calculator Websites

Numerical Calculations Nernst Equilibrium Potential Calculator The Download Scientific Diagram
Ppt Nernst Equation Powerpoint Presentation Free Download Id 3432521
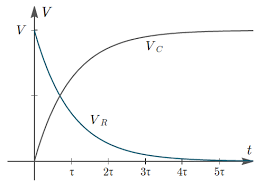
How To Calculate And Solve For Nernst Equation Corrosion Nickzom Blog
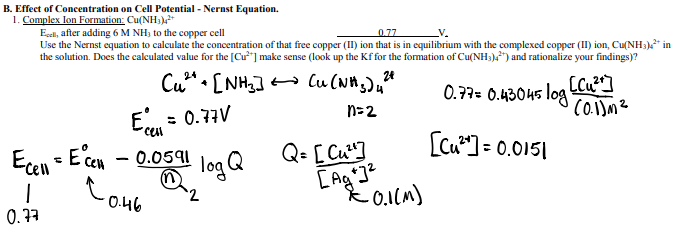
Solved V B Effect Of Concentration On Cell Potential Chegg Com

Concentration Cell Direction Of Electron Flow Nernst Equation Electrochemistry Mcat Content

Quiz Worksheet Nernst Equation Study Com